Electrolysis
Introduction
People who don't own boats tend to think sailing consists of moving across the water under sail or motor power, knowing where to safely drop an anchor, then pouring a drink as the sun sets in the west. Yes, those are part of the experience, but to successfully manage a boat, sailors have to think about any number of esoteric things. In this article I'll discuss just one of the many "gotchas" of boat ownership — electrolysis.
At its most basic, electrolysis describes how electricity can make metal ions move from one place to another. An example is electoplating, a technique to give an object consisting of one metal, a plating of another metal. For example, if you have an anchor chain, the external zinc coating that protects it was applied using a method called "galvanization" (although some modern galvanization procedures do away with the electric field).
Another, similar process is called "anodizing", commonly used to apply a protective layer and/or paint to aluminum.
All these methods use an electric field to transport ions from one place to another. It's very ingenious and very effecive.
On reading this description, an astute reader may wonder whether electrolysis happens naturally, perhaps in circumstances when it's not intended. Well, yes, that happens, and as it turns out, it's one of the more important problems in boat ownership.
To deal with this problem, most boats have one or more sacrificial zinc pieces attached to the boat's metal parts below the water line, because if a small electrical current is passing through water near the boat, zinc can be relied on to give up ions to electrolysis more readily than other kinds of metal, thus protecting more valuable parts like propellers and hulls.
Before we move on, here's the most basic description of electrolysis:
- Most water is conductive. Salt, fresh, every kind of water except extremely pure, conducts electricity.
- If a body of water carries an electrical current, most metals immersed in the water will emit ions (and dissolve over time) in response to the electrical current. This is called "electrolysis" or "galvanic corrosion."
- Given enough time, unless protective measures are taken, electrolysis will cause a metal object to dissolve away completely.
- One protective measure is to attach a piece of zinc to a metal object one wants to protect. The rationale is that zinc emits ions more readily and at a lower evergy level than the protected metal, thus preserving it.
- Another remedy is to electrically isolate a boat from small currents flowing through the water — this can be accomplished using a "galvanic isolator". A galvanic isolator protects against much, but not all, loss of metal to elecrolysis.
- Another source of electrolysis is a natural tendency for metal ions to move from one kind of metal to another in the presence of a conducting liquid. This is how many batteries work.
- The classic remedy for this kind of electrolysis (which doesn't require stray electrical currents to be flowing) is to avoid the use of dissimilar metals in boat construction.
That summarizes the basic issues, but there are some subtle examples of electrolysis within a boat that will reliably eat away various kinds of equipment, even in a boat equipped with a galvanic isolator and bristling with zincs.
Water Heater Blues
This season I analyzed one such subtlety involving my boat's water heater, and now that I am about to install my third water heater in ten years, I managed to figure out what's been eating them up.
Why should a water heater be an electrolysis issue? The heater has a safe, well-insulated alternating-current heating element that connects to shore power when available, and it has some plastic plumbing fittings that connect it to the boat's plumbing system. For safety reasons the heating element is well-insulated from the water and the tank's metal parts, and this insulation should also prevent electrolysis. Where's the problem?
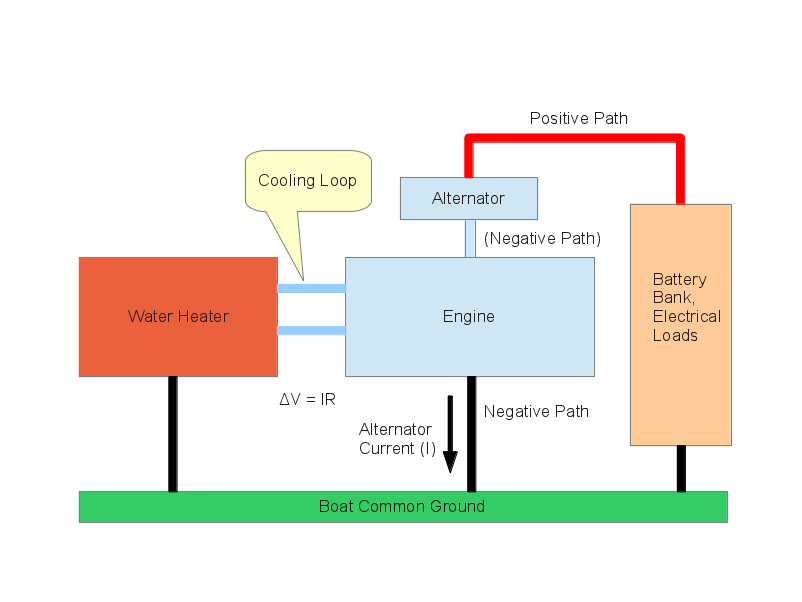
Well. As it happens, many modern boat water heaters also have a cooling-water loop connected to the boat's engine, to take advantage of the engine's rather hot cooling fluid, which carries heat that otherwise would go to waste. This arrangement seems relatively benign — after all, the engine is carefully grounded to the boat's grounding system, and it even has zincs of its own to minimize the chance of electrolysis.
But after watching several water heaters disintegrate, and especially when I noticed that the engine cooling-water loop fittings were the first parts to dissolve, I decided to think about the connection between the water heater and the engine. It was then that I realized something:
- While the engine is running, it spins an alternator that provides electrical power for the engine and charges the boat's batteries.
- In most cases, on most engines, the alternator's "negative" terminal is the alternator case. There's no separate conductor for the negative pole. This means the alternator's negative-terminal current passes through the alternator frame, then the engine, then through a thick cable, thence into the boat's grounding system.
- In most boats, a thick cable connects the engine to the boat's common ground point, where batteries and electrical equipment all connects.
- But, regardless of how thick this cable is, it has some resistance to current flow, and when the alternator is delivering significant current, that current creates a voltage across this cable (according to Ohm's Law: E = IR).
- This means while the engine is running and the alternator is spinning, the engine is elevated above ground voltage — it has a voltage different from that present at the water heater.
- Because the engine and the water heater are at two different voltage levels, the cooling fliud traveling between the engine and the water heater produces electrolysis and gradually dissolves the water heater's metal parts.
Remedies
After I realized the above was the reason for the water heater failures, I considered some remedies:
- Get a galvanic isolator just for the water heater — essentially, unground the water heater. But this won't work, because the stray current would flow from the engine, through the water heater, to the drinking-water system, and eventually to a suitable ground. This would only increase the number of places where electrolysis could occur.
- Ground the water heater to the engine instead of to the AC ground where it is normally connected, thus zeroing out the stray current. This won't work because (a) it violates electrical safety codes, and (b) as with the item above it only allows the stray current to follow a longer path and create elecrolysis in more places.
- Disconnect the engine cooling circuit from the water heater (loop it back onto itself). This works, but it sacrifices the convenience of heating water while running the engine.
- Buy a water heater equipped with an insulated engine cooling loop, a loop inside the water heater that's electrically insulated from the rest of the water heater, but that conducts heat reasonably well. This is a possible solution using modern heat-conducing ceramics, but at the moment, no such thing exists. It might be prohibitively expensive.
- Fit the engine with a special alternator that has a separate negative terminal not connected to the alternator frame, then run a separate negative cable from the alternator to the boat's common ground point. This works, but (a) not all engines can be fitted with a compatible isolated-terminal alternator, and (b) such alternators tend to run hotter and may overheat sooner than a conventional design.
Several years ago I acquired a special alternator that had a separate negative terminal. I acquired it for a different reason, one unrelated to the water heater (I wanted to charge my big battery bank more efficiently). It seemed to work all right, until the day it caught fire.
As I cleared the smoke from my boat's cabin, I decided that the advantages of a separate-terminal alternator were more than outweighed by the prospect of catching my boat on fire, so I gave up on that "remedy".
Conclusion
Boats have some issues that make them much more complicated to own and operate than cars, and most boat owners have no knowledge of matters like the one discussed on this page. Even people who work in boat yards don't necessarily understand these issues.
I'm a former NASA engineer, some of my electronic designs flew on the Space Shuttle, and even I need to think hard (or harder) about certain boat issues like this one. It took me a while to realize there was a connection between the most common kind of alternator and dissolving water heaters.
And the bottom line for this problem is — there's no easy solution. For most boats and most owners, if you really care about making your water heater last more than a few seasons, I recommend that you disconnect the engine cooling loop from the heater (loop it back onto itself) and give up the convenience of heating water while underway.

 Share This Page
Share This Page

 Share This Page
Share This Page



 Share This Page
Share This Page